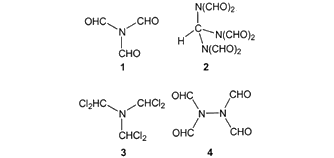
Gold(I)-Mediated Cycloisomerization/Cycloaddition Enables Bioinspired Syntheses of Neonectrolides B-E and Analogues. - Abstract - Europe PMC

Summary of the molecular structures and field-effect mobility measured... | Download Scientific Diagram
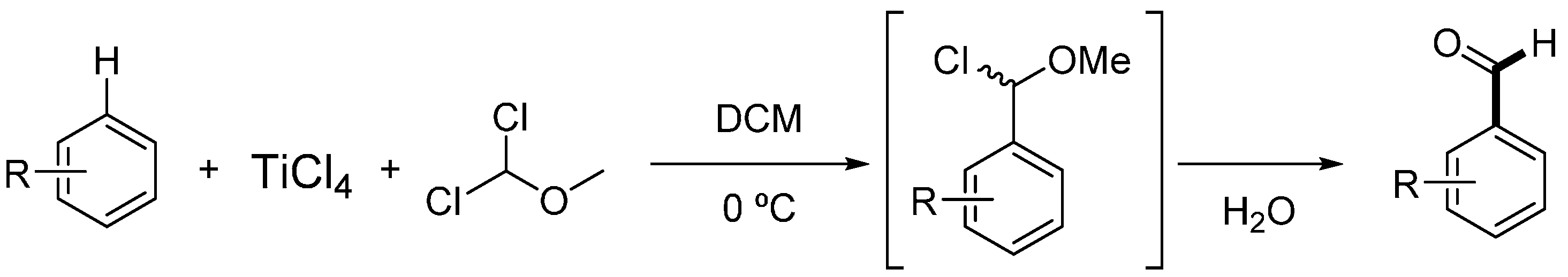
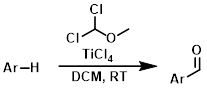
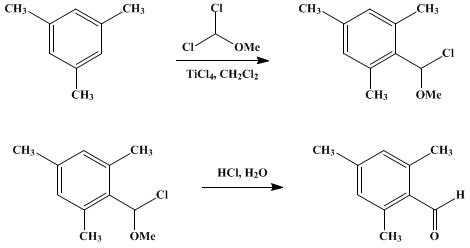
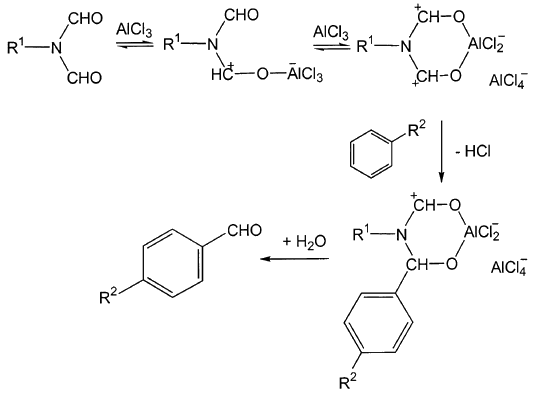
Molecules | Free Full-Text | Formylation of Electron-Rich Aromatic Rings Mediated by Dichloromethyl Methyl Ether and TiCl4: Scope and Limitations | HTML

Metal-free selective aryl C–H formylation co-controlled by 1,2,3-triazole and hydroxyl using DMSO as formyl source | SpringerLink

Metal-free selective aryl C–H formylation co-controlled by 1,2,3-triazole and hydroxyl using DMSO as formyl source | SpringerLink

Palladium-catalyzed C-H formylation of electron-rich heteroarenes through radical dichloromethylation - ScienceDirect

Efficient preparation of dichloromethyl alkyl ethers and their application in the formylation of aromatic compounds: Scope and limitations - ScienceDirect

Efficient preparation of dichloromethyl alkyl ethers and their application in the formylation of aromatic compounds: Scope and limitations - ScienceDirect
![Redox neutral [4+2] benzannulation of dienals and tertiary enaminones for benzaldehyde synthesis - Chemical Communications (RSC Publishing) DOI:10.1039/C8CC03514H Redox neutral [4+2] benzannulation of dienals and tertiary enaminones for benzaldehyde synthesis - Chemical Communications (RSC Publishing) DOI:10.1039/C8CC03514H](https://pubs.rsc.org/image/article/2018/CC/c8cc03514h/c8cc03514h-f1_hi-res.gif)
Redox neutral [4+2] benzannulation of dienals and tertiary enaminones for benzaldehyde synthesis - Chemical Communications (RSC Publishing) DOI:10.1039/C8CC03514H
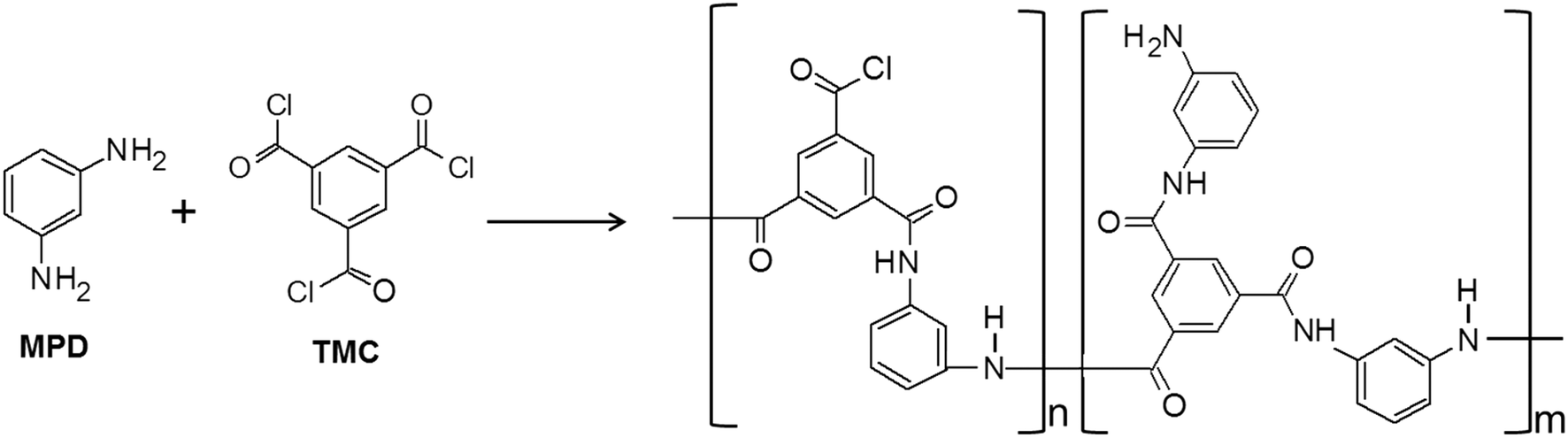
Influence of nanoparticle inclusions on the performance of reverse osmosis membranes - Environmental Science: Water Research & Technology (RSC Publishing) DOI:10.1039/C7EW00420F

Efficient synthesis of polymethoxyselenoflavones via regioselective direct C–H arylation of selenochromones - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C7OB00118E

Metal-free selective aryl C–H formylation co-controlled by 1,2,3-triazole and hydroxyl using DMSO as formyl source | SpringerLink
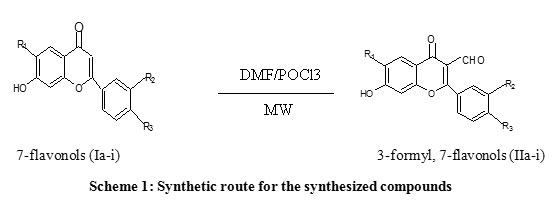
Anti-Inflammatory Effects of 3-Formyl, 7-Flavonols Derivatives by Microwave Enhanced Chemistry Assisted – Vilsmeier Haack Synthesis | Biomedical and Pharmacology Journal