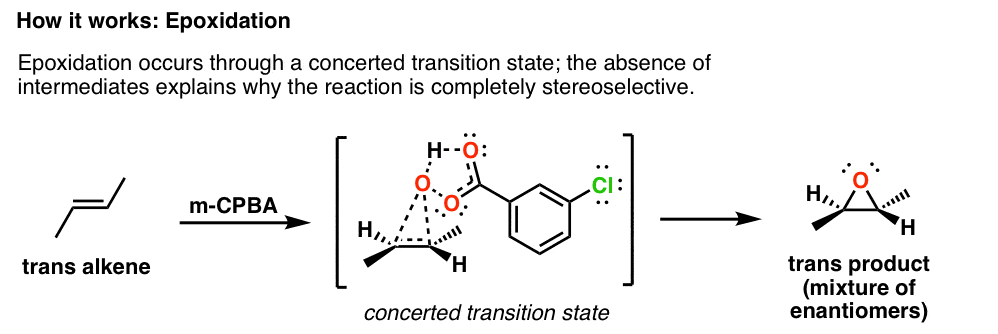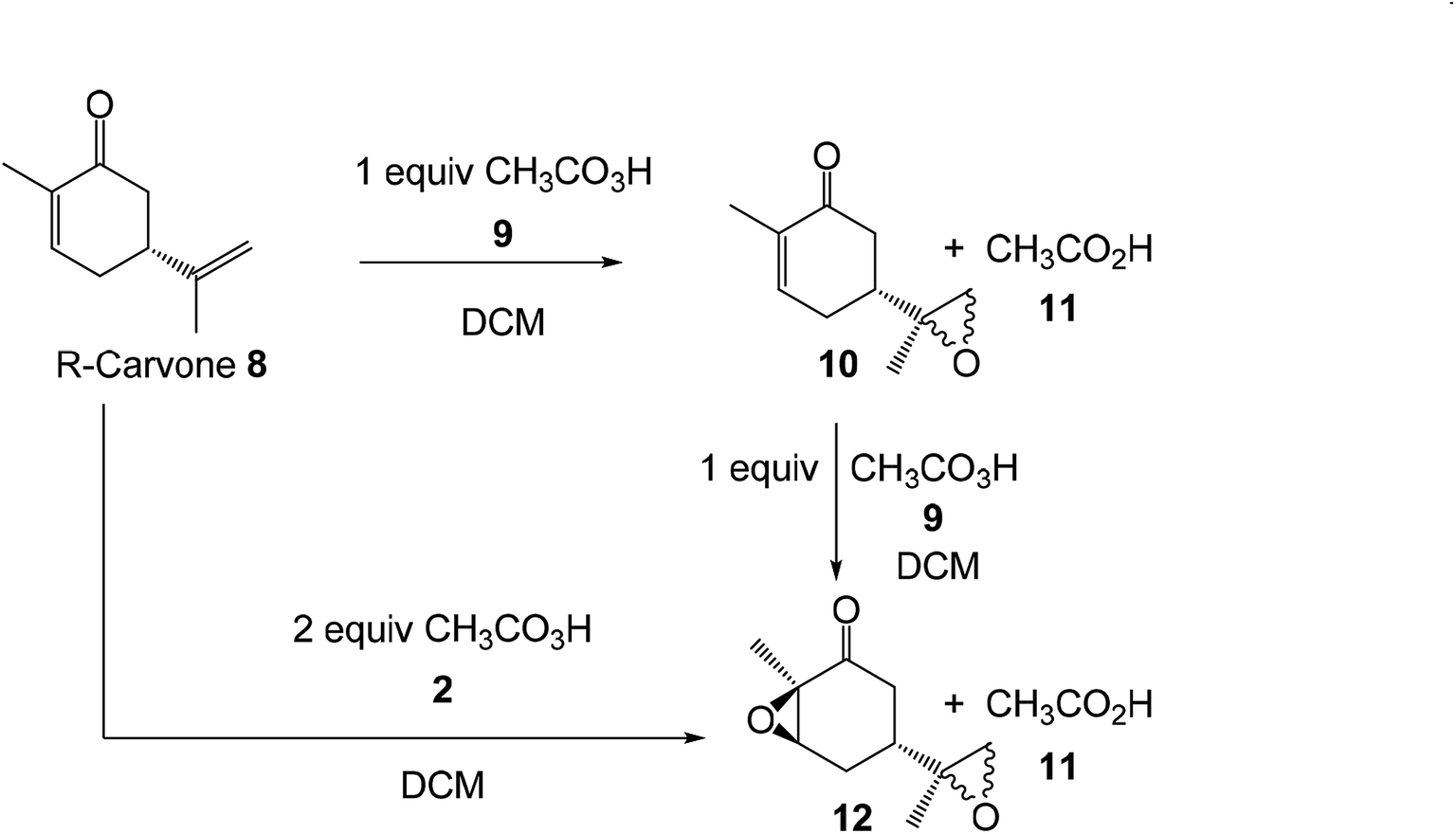
A molecular electron density theory study of the mechanism, chemo- and stereoselectivity of the epoxidation reaction of R-carvone with peracetic acid - RSC Advances (RSC Publishing)
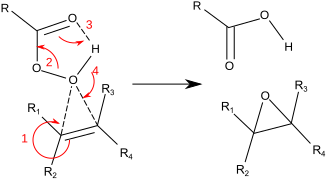
How can I explain the mechanism of acid-catalyzed epoxidation of alkenes with peroxy acids? | Socratic

Why does the opening of an epoxide occur via an Sn2 like mechanism when using a methanoate ion as a nucleophile and methanol as a solvent? - Chemistry Stack Exchange

![Epoxidation [RCO3H] - ChemistryScore Epoxidation [RCO3H] - ChemistryScore](https://mk0chemistrysco84nst.kinstacdn.com/wp-content/uploads/2019/11/Epoxidation1-1024x439.png)


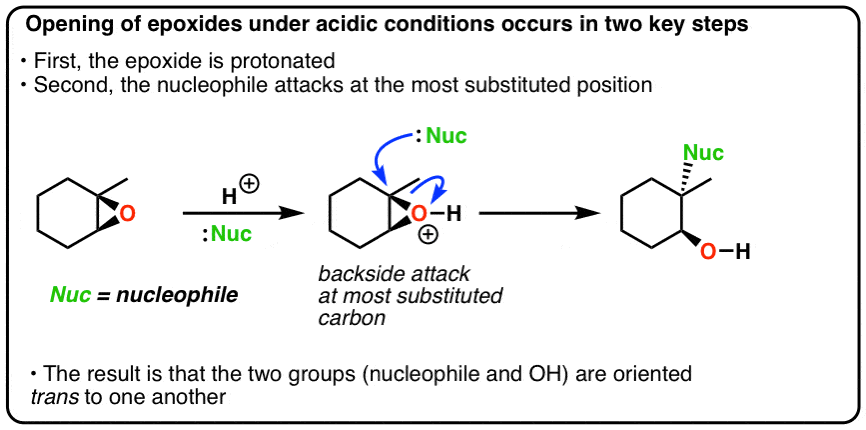

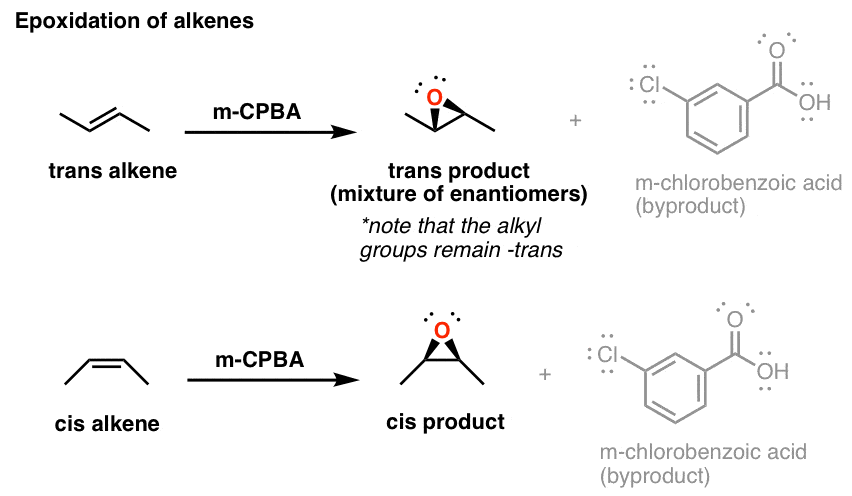


.png?revision=1)